Contextual Framework
Posted: March 21st, 2015 | Author: admin | Filed under: Contextual framework, Publications | No Comments »The candle as object and everyday item
A candle is composed of solid wax surrounding a wick, which, when lit, produces a certain amount of light and heat. Like oil lamps, candles were mainly used to shed light in otherwise dark homes. The original material used for making candle wax was the body fat of animals – also known as tallow – which has an intense smell when burned. In the nineteenth century a process was developed to refine tallow with alkali and sulphuric acid to produce stearin. While still produced from animal fat, stearin was less greasy and smelly than ordinary tallow.
Candle idioms and phrases
In everyday language, candles are often referred to in the context of idioms and commonly used phrases. For example, to “burn the candle at both ends” implies that doing something twice as much leads to quicker deterioration. To say that one feels “burned out” is used to express that one feels deprived of energy. The phrase “the brighter candle burns quicker” communicates the idea that the more energy one spends (e.g. by living life in an extreme or intense way) the quicker everything will come to an end – similar to the expression “live fast, die young”. From these examples, it becomes clear that the candle is often used as a symbol for human life in general, and more particularly for vital energy, as well as for specific amounts of energy and time.
The candle as time-keeping device
In addition to their main purpose of producing light, candles have also been used as time-measurement devices, known as “candle clocks”. A candle clock is a device consisting of a candle holder with regular markings in which a candle is inserted. As the candle burns down, the passage of time is measured based on the material consumption of the candle, as indicated by the markings. Candle clocks are “elementary clocks”: devices that measure time based on the behaviour of physical materials such as fire, water or sand. Contrary to other time-measurement devices, a candle clock can be used only once. One historically significant use of candle clocks was their use by monks in the Middle Ages, who used them to structure the day in the monastery, and served particularly to ensure the regularity of the praying hours.
The candle as vanitas symbol
The candle is one of the classical “vanitas symbols”. Vanitas symbols are objects such as skulls, fading flowers, perishable foods or timepieces which have been depicted in still-life paintings and mediaeval funerary art to remind the viewer of the transient nature of life and the passage of time. In general, a burning candle with its vivid yet elusive flame often stands for human life or the soul, while an extinguishing candle often symbolizes death. Contrary to other vanitas symbols such as the skull, the burning candle is not just a static object but also indicates the passage of time during process of burning down.
Fig. 7. Left: candle clock; right: The Penitent Magdalen (Magdalena Wrightsman), 1625–1650, Georges de La Tour.
The candle as religious symbol
The candle is also an important object and symbol in many religious beliefs. In the Christian context, the candle flame stands for the eternal presence of the light of God. Also, it is often associated with eternal life and the immortality of the soul. The lighting of the Easter candle symbolizes the overcoming of mortal death by Jesus. In other religions such as Buddhism, a burning candle may also stand for a general notion of spiritual enlightenment.
A theory of combustion
While the controlled use of fire has accompanied human evolution from early on, the appearance of fire remained an inexplicable phenomenon or was understood as magical or divine. In the fifteenth century, Leonardo da Vinci proposed that one part of air is used up during combustion while the remaining air extinguishes the flame, but it was only about 250 years ago that the process of combustion was first described as a chemical reaction. From the seventeenth to the eighteenth centuries, the so-called “phlogiston theory” claimed that a fiery material is produced during the burning process. Only in the second half of the eighteenth century did Antoine de Lavoisier explain, based on his experiments on the calcination of metals and the availability of precise scales, that combustion is a chemical reaction in which oxygen is absorbed. The “oxygen theory of combustion” as well as Lavoisier’s Traité élémentaire de Chimie marked the shift from alchemy to “modern chemistry” as an exact science based on methodical experimentation and precise measurement.
 Fig. 8. Michael Faraday performing an experimental lecture at the theatre of the Royal Institution in London.
Fig. 8. Michael Faraday performing an experimental lecture at the theatre of the Royal Institution in London.
The chemical history of a candle
In 1860, the English scientist Michael Faraday performed a series of six experimental lectures entitled The Chemical History of a Candle in which he investigated the chemical and physical properties of candle flames. This lecture series was part of the so-called Christmas lectures for young people, which were traditionally held each year at the Royal Institution in London with the purpose of presenting science to the general public. Faraday used the candle as a starting point to explore and demonstrate the nature of combustion; in effect, Faraday’s lectures transformed the candle from a simple everyday item into an object of scientific investigation. In the last of these lectures Faraday also elaborated on respiration and its analogy to the burning of a candle: In every one of us there is a living process of combustion going on very similar to that of a candle, and I must try to make that plain to you. For it is not merely true in a poetical sense – the relation of the life of man to a taper (wick); and if you will follow, I think I can make this clear.. [Faraday, 1861, p. 173] The relation between combustion and metabolism (as pointed out by Faraday and previously in the context of the phlogiston theory) led to the recognition that the metabolism of living organisms and combustion can be understood in terms of fundamentally related chemical processes.
Nineteenth century physiology and the study of respiration
Meanwhile, in the physiological laboratory of the nineteenth century, a number of respiration apparatuses were built to scientifically analyse and quantify the breathing process. These experimental setups, which could consist, for example, of the breathing organism, the ticking of the metronome, the experimenter´s eye as well as handwritten entries in tables and laboratory books can be described as heterogeneous arrangements in between organisms and clocks [Schmidgen, 2005a, p. 246]. In parallel, the physiological time of organisms became an important parameter to be measured and described.
Homeostasis
The development of physiology picked up rapidly in the nineteenth century, with new concepts such as “homeostasis” being introduced. While the concept of homeostasis originally referred to the process of maintenance of a state of equilibrium in the live organism, it was later also applied more generally to selfregulatory systems. In the most general sense, it therefore refers to the regulation of equilibrium through a control mechanism comprising some sort of sensor, an effector and negative feedback.
Metabolic rate and respiratory quotient
The metabolic rate indicates the speed of metabolism or, in other words, the speed at which chemical reactions related to metabolism take place in an organism. It is linked to the rate at which a body consumes oxygen and the heat produced in the process. The respiratory quotient indicates the rate of respiration and is used when calculating the metabolic rate. It is calculated from the ratio of oxygen consumption and carbon dioxide production. The measurement can be performed in a device called a respirometer or oxygraph, which mainly consists of a closed container comprising the breathing specimen.
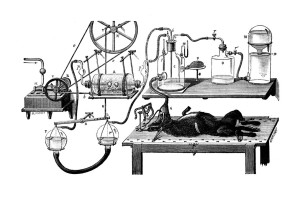
Fig. 9. Apparatus for the study of respiration products by Jolyet and Regnard (1879). By turning a valve the animal´s respiratory system could be directly connected with an oxygen bottle, which is linked to a device producing constant pressure. The outgoing air could be analysed for carbonic acid and the amount of oxygen used could be determined.
Ageing research and the “rate-of-living theory”
There are a number of theories of why organisms age; the rate-of-living theory is one such theory, which claims that the life span of organisms is proportional to their energy turnover or metabolic rate: the higher the metabolic rate, the faster the biochemical activity and thus the faster an organism will age. The rate-of-living theory was one of the very first theories of ageing, and had been widely accepted throughout most of the twentieth century. It was originally inspired by the observation that larger organisms have a lower metabolic rate and at the same time often live longer. Numerous experiments on model organisms have shown evidence for this theory: for example, it has famously been demonstrated that calorie restriction could significantly prolong the life span of rodents. Further variations of the theory point not only to calorie intake, but also to heart rate and oxygen consumption as important factors involved in metabolic rate and ageing. Nowadays, while the rate-of-living theory has become more controversial, approaches such as calorie restriction are still current topics in ageing research and continue to be explored.
Life extension
Organisms and systems experience time as a function of their physiological and material properties. As such, it is possible to confer a characteristic life span to a given organism. However, it has become increasingly common to manipulate the time of living organisms. Indeed, the life span of laboratory animals has been frequently manipulated in the lab, while human life span has been increasingly extended in the last 200 years via advances in medical and biotechnological interventions. Life expectancy first took an important leap forward thanks to better hygienic measures and standards of living, followed by the large-scale availability of pharmaceutical products in the twentieth century. Now, a new era characterized by pharmaceutical intervention and the increasing personalization of medicine can be envisaged. Additionally, responsibility for the direction of one´s health is shifting to the individual, with a focus on personal enhancement, lifestyle and prevention – including physical exercise, the intake of dietary supplements and nutrition. New possibilities of medical interventions, where body parts, organs and tissues are repaired, replaced or exchanged, promise to extend human life even further.